Research Area B
Publications 2008
22-Jun-2009
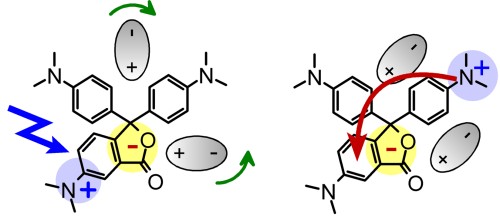
The intramolecular electron-transfer reaction in crystal violet lactone in polar aprotic solvents is studied with femtosecond transient absorption spectroscopy. The initially excited charge transfer state 1CTA is rapidly converted into a highly polar charge transfer state 1CTB. This ultrafast electron transfer is seen as a solventdependent dual fluorescence in ... READ MORE
15-Dec-2008
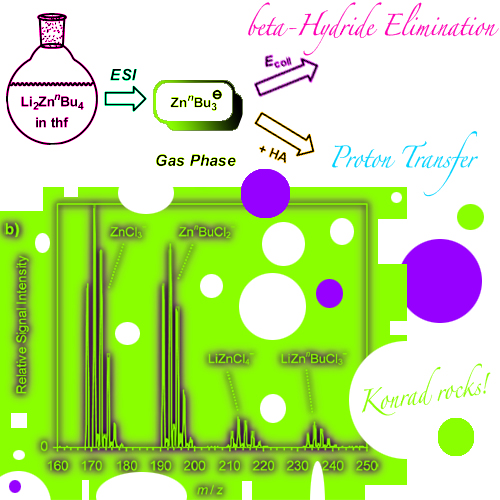
Electrospray ionization (ESI) of mixtures of organolithium compounds and zinc chloride in tetrahydrofuran produced manifold mono- and polynuclear organozincate anions. Formation of the latter is strongly favored by the incorporation of chloride ligands, which apparently adopt bridging binding modes. Analysis of LinBu/ZnCl2 solutions at different concentrations ... READ MORE
04-Nov-2008
3,6-Dichloropyridazine undergoes a smooth metallation using (tmp)_2 Zn 2MgCl_2 2LiCl. The resulting bis-organozinc species react with various electrophiles; subsequent functionalization via a second metallation proceeds readily; further reactions with hydrazine lead to highly substituted pyrazolo[3,4-c]pyridazines derivatives. ... READ MORE
28-Jul-2008
The preparation of complex polyfunctional organometallic reagents is of pivotal interest in organic synthesis.[1] Recently, functionalized organomagnesium reagents have become accessible through a halogen/magnesium exchange[2] or a directed metalation.[3] During these studies it became apparent that aryl magnesium halides are compatible with important functional ... READ MORE
17-Jul-2008
A wide range of polyfunctional aryl, heteroaryl, alkyl, and benzylic zinc reagents were coupled with unsaturated aryl halides bearing an acidic NH or OH proton, using Pd(OAc)_2 (1 mol %) and S-Phos (2 mol %) as catalyst without the need of protecting groups. A similar nickel-catalyzed reaction is described. The relative kinetic basicity of organozinc compounds as ... READ MORE
05-Jun-2008
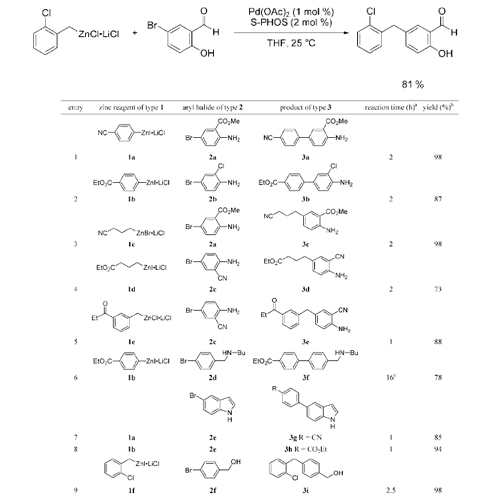
A wide range of polyfunctional aryl, heteroaryl, alkyl, and benzylic zinc reagents were coupled with unsaturated halides bearing an acidic NH or OH function, using Pd(OAc)2 (1 mol %) and S-Phos (2 mol %) as catalyst without the need of protecting groups.
... READ MORE30-May-2008
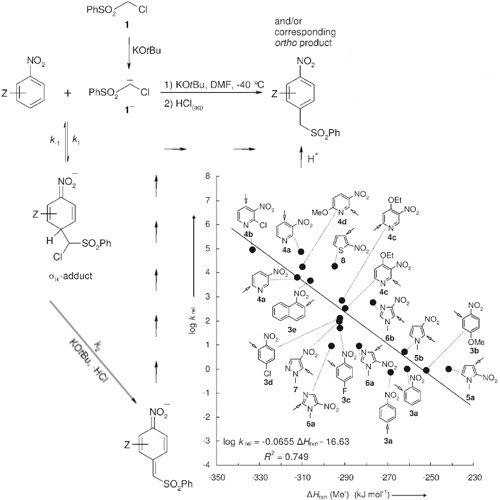
The relative rate constants for the vicarious nucleophilic substitution (VNS) of the anion of chloromethyl phenyl sulfone with a variety of nitroheteroarenes, for example, nitropyridines, nitropyrroles, nitroimidazoles, 2-nitrothiophene, and -nitropyrazole, have been determined by competition experiments. It was shown that nitropyridines are approximately four ... READ MORE
21-May-2008
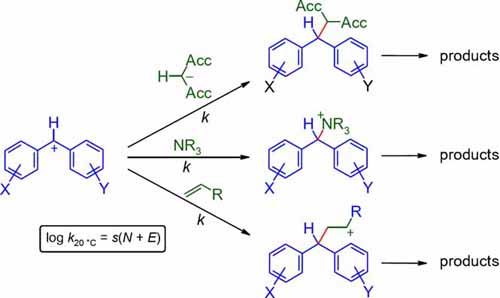
Comprehensive nucleophilicity scales including π-, n- and σ-nucleophiles have been constructed using benzhydrylium ions and structurally related quinone methides as reference electrophiles. It is shown how the correlation (Eqn (1)) log k_(20°C) = s(E + N), where s and N are nucleophile-specific parameters and E is an electrophile-specific parameter, has ... READ MORE
09-Apr-2008
Using regioselective cuprations (via magnesiations), various primary, secondary and tertiary aminated pyrimidine and purine derivatives were prepared by the oxidative coupling of lithium amidocuprates using chloranil. DNA and RNA units such as aminated uracil or thymine, and adenine, as well as a CDK inhibitor, purvalanol A, were all obtained under mild conditions ... READ MORE
28-Mar-2008
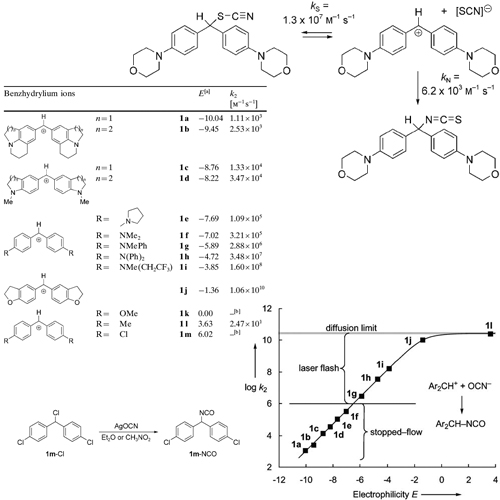
The cyanate anion is an ambident nucleophile, which may react with electrophiles either at the oxygen terminus to yield alkyl cyanates or at the nitrogen terminus to yield isocyanates. Because the charge density is higher at the more electronegativeoxygen center while the larger HOMO coefficient is at nitrogen, the concept of charge and orbital control predicts ... READ MORE
19-Mar-2008
Cinnamylzinc reagents react with cyclic and acyclic r-chiral ketones under very mild conditions (-78 °C, 1 h), yielding the corresponding homoallylic alcohols bearing three adjacent stereocenters with high diastereoselectivity. An extension of this reaction to enantioenriched r-chiral ketones is also described. ... READ MORE
06-Mar-2008
Readily prepared b-silyl substituted crotylzinc reagents undergo highly selective allylation of carbonyl compounds leading to syn-homoallylic alcohols. ... READ MORE
06-Dec-2007
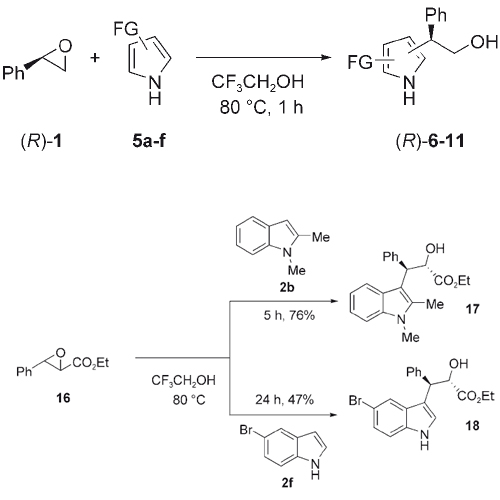
Aliphatic and aromatic epoxides react regio- and stereoselectively with indoles and pyrroles in 2,2,2-trifluoroethanol without the use of a catalyst or any other additive. While aromatic epoxides are selectively attacked at the benzylic position, aliphatic epoxides react at the less-substituted position. Chiral epoxides react with >99% ee (ee=enantiomeric ... READ MORE
23-Nov-2007
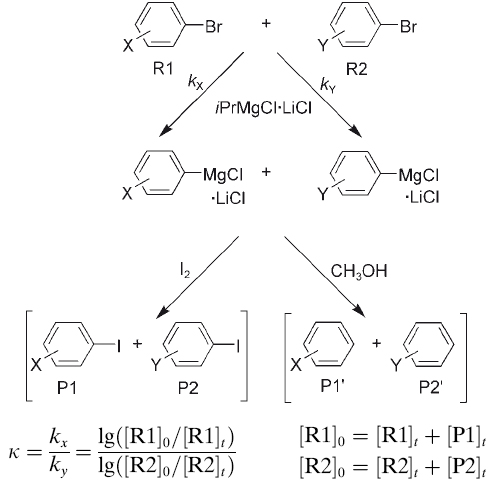
The accessibility of functionalized Grignard reagents has opened new dimensions in organic synthesis. One of us has previously demonstrated that treatment of substituted bromoarenes with iPrMgCl·LiCl provides a straightforward access to functionalized aryl Grignard reagents by bromine–magnesium exchange. In accord with the suggested mechanism for these ... READ MORE


