An unlocking/relocking barrier in conformational fluctuations in villin headpiece subdomain
16-Mar-2010
PNAS, 2010, 107, No. 11, 4955-4960 published on 16.03.2010
PNAS
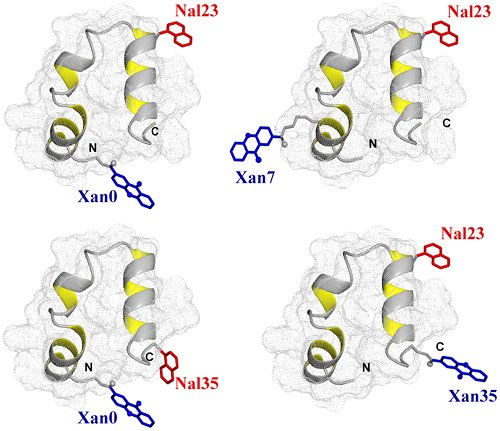
A reversible structural unlocking reaction, in which the close-packed van der Waals interactions break cooperatively, has been found for the villin headpiece subdomain (HP35) using triplet-triplet-energy transfer to monitor conformational fluctuations from equilibrium. Unlocking is associated with an unfavorable enthalpy change which is nearly compensated in free energy by the entropy change. The unlocking reaction has a time constant of about 1 μs at 5 °C and is enthalpy-limited with an activation energy of 32 ± 1 kJ/mol and a large Arrhenius preexponential factor of A = 7.5 × 1011 s-1. In the unlocked state a fast local conformational fluctuation with a time constant of 170 ns and a low activation barrier of 17 ± 1 kJ/mol leads to unfolding of the C-terminal helix and to its undocking from the core. On a much slower time scale, global unfolding occurs from the unlocked state. These results suggest that native protein structures are locked into conformations with low amplitude motions. Large scale motions and global unfolding require an initial structural unlocking step leading to a state with properties of a dry molten globule. The experiments additionally yielded information on the dynamics of loop formation between different positions in unfolded HP35. Comparison of the results with dynamics in unstructured model peptides indicates slightly decelerated kinetics of local loop formation in the C-terminal region which points at residual, nonrandom structure. Dynamics of long-range loop formation, in contrast, are not influenced by residual structure, which argues against unfolded state properties as molecular origin for ultrafast folding of HP35.