Unraveling the flavin-catalyzed photooxidation of benzylic alcohol with transient absorption spectroscopy from sub-pico- to microseconds
Phys. Chem. Chem. Phys., 2011, 13, 8869-8880 published on 01.04.2011
Phys. Chem. Chem. Phys.
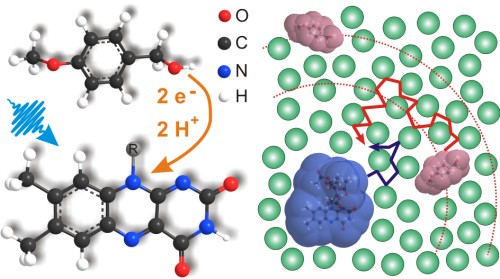
Flavin-mediated photooxidations have been described for applications in synthetic organic chemistry for some time and are claimed to be a route to the use of solar energy. We present a detailed investigation of the involved photophysical and photochemical steps in methoxybenzyl alcohol oxidation on a timescale ranging from sub-picoseconds to tens of microseconds. The results establish the flavin triplet state as the key intermediate for the photooxidation. The initial step is an electron transfer from the alcohol to the triplet state of the flavin catalyst with 3kET ~ 2x107 M-1s-1, followed by a proton transfer in ~ 6 µs. In contrast, the electron transfer involving the singlet state of flavin is a loss channel. It is followed by rapid charge recombination (τ = 50 ps) without significant product formation as seen when flavin is dissolved in pure benzylic alcohol. In dilute acetonitrile/water solutions of flavin and alcohol the electron transfer is mostly controlled by diffusion, though at high substrate concentrations > 100 mM we also find a considerable contribution from preassociated flavin-alcohol-aggregates. The model including a productive triplet channel and a competing singlet loss channel is confirmed by the course of the photooxidation quantum yield as a function of substrate concentration: We find a maximum quantum yield of 3% at 25 mM of benzylic alcohol and significantly smaller values for both higher and lower alcohol concentrations. The observations indicate the importance to perform flavin photooxidations at optimized substrate concentrations to achieve high quantum efficiencies and provide directions for the design of flavin photo-catalysts with improved performance.