Regio- and Stereoselective Ring-Opening Reactions of Epoxides with Indoles and Pyrroles in 2,2,2-Trifluoroethanol
06-Dec-2007
Chemistry, 2008, 14, 1638-47 published on 06.12.2007
Chemistry; online article
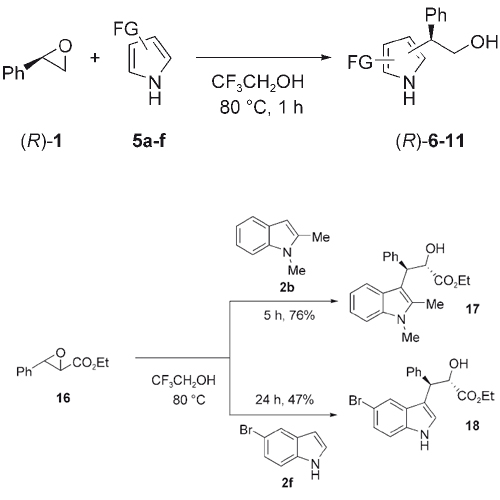
Aliphatic and aromatic epoxides react regio- and stereoselectively with indoles and pyrroles in 2,2,2-trifluoroethanol without the use of a catalyst or any other additive. While aromatic epoxides are selectively attacked at the benzylic position, aliphatic epoxides react at the less-substituted position. Chiral epoxides react with >99% ee (ee=enantiomeric excess).