Quantification of the Ambident Electrophilicities of Halogen-Substituted Quinones
23-Jul-2014
J. Am. Chem. Soc., 2014, 136 (32), pp 11499–11512 published on 23.07.2014
J. Am. Chem. Soc.
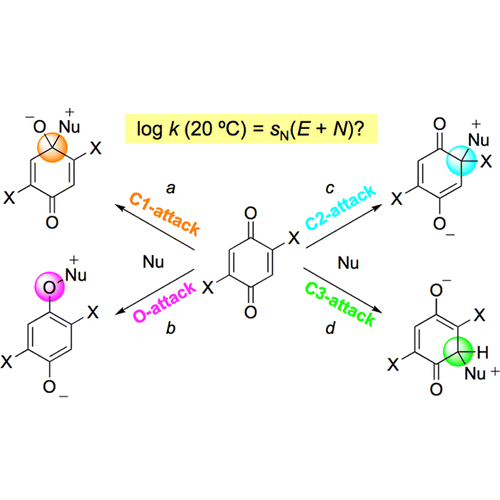
Kinetics and mechanisms of the reactions of p-quinone, 2,5-dichloro-p-quinone, 2,3,4,5-tetrachloro-p-quinone (chloranil), 2,3,4,5-tetrafluoro-p-quinone (fluoranil), and 3,4,5,6-tetrachloro-o-quinone with π-nucleophiles (siloxyalkenes, enamines) and amines have been investigated. Products arising from nucleophilic attack at all conceivable sites, that is, at C and O of the carbonyl groups (pathways a, b) as well as at halogenated and nonhalogenated conjugate positions (pathways c, d), were observed. The partial rate constants for the C-attack pathways (a, c, d), which are derived from the photometrically determined second-order rate constants and the product ratios followed the linear free energy relationship log k (20 °C) = sN(E + N) (Mayr, H.; J. Am. Chem. Soc. 2001, 123, 9500−9512). It was, therefore, possible to calculate the electrophilicity parameters E of the different positions of the quinones from log k (20 °C) and the N and sN parameters of the nucleophilic reaction partners, which have previously been derived from their reactions with benzhydrylium ions. Almost all rate constants for the C-attack pathways (a, c, d) were considerably larger than those calculated for the corresponding SET processes, indicating the operation of polar mechanisms. SET mechanisms may only account for the formation of the products formed via O-attack. With the E parameters determined in this work, it is now possible to predict rate constants for the reactions of these quinones with a large variety of nucleophiles and, thus, envisage unprecedented reactions of quinones.