Photolytic Generation of Benzhydryl Cations and Radicals from Quaternary Phosphonium Salts: How Highly Reactive Carbocations Survive Their First Nanoseconds
11-Oct-2012
J. Am. Chem. Soc., 2012, 134 (28), 11481–11494 published on 15.05.2012
J.Am.Chem.Soc.
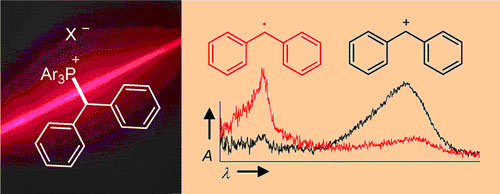
UV irradiation (266 or 280 nm) of benzhydryl triarylphosphonium salts ... UV irradiation (266 or 280 nm) of benzhydryl triarylphosphonium salts Ar2CH–PAr3+ X– yields benzhydryl cations Ar2CH+ and/or benzhydryl radicals Ar2CH·. Efficiency and mechanism of the photo-cleavage were studied by nanosecond laser flash photolysis and by ultrafast spectroscopy with a state-of-the-art femtosecond transient spectrometer. The influence of the photo-electrofuge (Ar2CH+), the photo-nucleofuge (PPh3 or P(p-Cl-C6H4)3), the counterion (X– = BF4–, SbF6–, Cl–, or Br–) and the solvent (CH2Cl2 or CH3CN) was investigated. Photogeneration of carbocations from Ar2CH–PAr3+ BF4– or SbF6– is considerably more efficient than from typical neutral precursors (e.g., benzhydryl chlorides or bromides). The photochemistry of phosphonium salts is controlled by the degree of ion pairing which depends on the solvent and the concentration of the phosphonium salts. High yields of carbocations are obtained by photolyses of phosphonium salts with complex counterions (X– = BF4– or SbF6–), while photolyses of phosphonium halides Ar2CH–PPh3+ X– (X– = Cl– or Br–) in CH2Cl2 yield benzhydryl radicals Ar2CH· due to photo-electron transfer in the excited phosphonium halide ion pair. At low concentrations in CH3CN, the precursor salts are mostly unpaired, and the photo-cleavage mechanism is independent of the nature of the counter-anions. Dichloromethane is better suited for generating the more reactive benzhydryl cations than the more polar and more nucleophilic solvents CH3CN or CF3CH2OH. Efficient photo-generation of the most reactive benzhydryl cations (3,5-F2-C6H3)2CH+ and (4-(CF3)-C6H4)2CH+ was only achieved using the photo-leaving group P(p-Cl-C6H4)3 and the counter-anion SbF6– in CH2Cl2. The lifetimes of the photogenerated benzhydryl cations depend greatly on the decay mechanisms, which can be reactions with the solvent, with the photo-leaving group PAr3, or with the counter-anion X– of the precursor salt. However, the nature of the photo-leaving group and the counterion of the precursor phosphonium salt do not affect the rates of the reactions of the obtained benzhydryl cations towards added nucleophiles. The method presented in this work allows to generate a wide range of donor- and acceptor-substituted benzhydryl cations Ar2CH+ for the purpose of studying their electrophilic reactivities.