Nucleophilicity Parameters for Phosphoryl-Stabilized Carbanions and Phosphorus Ylides: Implications for Wittig and Related Olefination Reactions
23-Dec-2008
J. Am. Chem. Soc., 2009, 131, 704–714 published on 23.12.2008
J. Am. Chem. Soc.
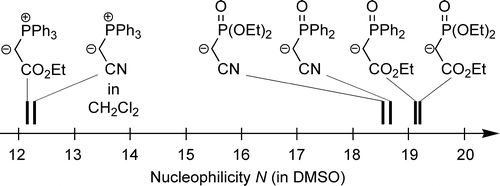
The kinetics of the reactions of four phosphoryl-stabilized carbanions 1a−d and four phosphorus ylides 1e−h with benzhydrylium ions 2a−h and structurally related quinone methides 2i−m have been determined by UV−vis spectroscopy. The second-order rate constants (k) correlated linearly with the electrophilicity parameters E of 2a−m, as required by the correlation log k = s(N + E) (J. Am. Chem. Soc.2001, 123, 9500−9521), allowing us to calculate the nucleophile-specific parameters N and s for phosphoryl-substituted carbanions and phosphorus ylides. In this way, a direct comparison of the nucleophilic reactivities of Horner−Wadsworth−Emmons carbanions and Wittig ylides became possible. Ph2PO- and (EtO)2PO-substituted carbanions are found to show similar reactivities toward Michael acceptors, which are 104−105 times higher than those of analogously substituted phosphorus ylides. The relative reactivities of these nucleophiles toward benzaldehydes differ significantly from those toward carbocations and Michael acceptors, in accordance with a concerted [2 + 2] cycloaddition being the initial step of these olefinations reactions. Effects of the counterion (K+, Na+, or Li+) on the nucleophilicities of the phosphoryl-stabilized carbanions in DMSO have been studied. Whereas the effects of K+ and Na+ are almost negligible for all types of carbanions investigated, Li+ coordination reduces the reactivities of phosphonate-substituted acetic ester anions (1a) by a factor of 102 while the reactivities of phosphonate-substituted acetonitrile anions (1b) remain almost unaffected.