Nucleophilic Reactivities of Hydrazines and Amines: The Futile Search for the α-Effect in Hydrazine Reactivities
28-Nov-2012
J. Org. Chem., 2012, 77, 8142-8155 published on 27.08.2012
J.Org.Chem.
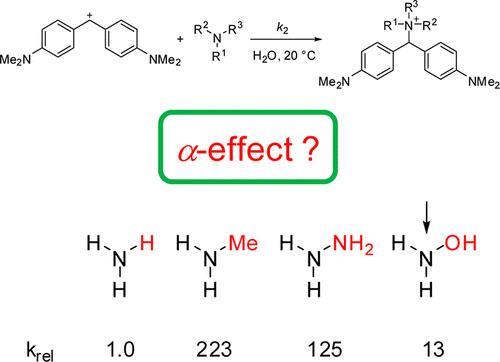
The kinetics of the reactions of amines, hydrazines, hydrazides, and hydroxylamines with benzhydrylium ions and quinone methides were studied in acetonitrile and water by UV–vis spectroscopy, using conventional spectrometers and stopped-flow and laser-flash techniques. From the second-order rate constants k2 of these reactions, the nucleophilicity parameters N and sN were determined according to the linear free energy relationship log k2 = sN(N + E). While methyl groups increase the reactivities of the α-position of hydrazines, they decrease the reactivities of the β-position. Despite the 102 times lower reactivities of amines and hydrazines in water than in acetonitrile, the relative reactivities of differently substituted amines and hydrazines are almost identical in the two solvents. In both solvents hydrazine has a reactivity similar to that of methylamine. This observation implies that replacement of one hydrogen in ammonia by Me increases the nucleophilicity more than introduction of an amino group, if one takes into account that hydrazine has two reactive centers. Plots of log k2 versus the corresponding equilibrium constants (log K) or Brønsted basicities (pKaH) do not show enhanced nucleophilicities (α-effect) for either hydrazines or hydroxylamine relative to alkylamines.